Levofloxacin(レボフロキサシン)特許の有効性は?(カナダ最高裁上告棄却): Supreme Court of Canada Docket No. 32200
Levaquin(一般名:levofloxacin(レボフロキサシン)、ニューキノロン系抗生物質、日本での販売名はクラビット)の後発品をカナダで販売するNovopharm(Tevaの子会社)に対して、その特許権者である第一製薬(現・第一三共)とそのライセンシーであるJanssen-Ortho(Johnson&Johnsonの子会社)が特許権(CA1,304,080)侵害を主張。
カナダ最高裁がNovopharmの上告を棄却したことによって、特許は有効であって特許権侵害であるとした連邦控訴裁判所の判断が確定した。
カナダ最高裁websiteより:
Summary
Intellectual property – Patents – Medicines – Whether the law of selection patents confers a second monopoly to a compound on the basis of properties that are the same as those of the genus from which the compound was selected – Whether “motivation” to select a previously disclosed compound should be imported into and given central importance in the test for obviousness – Whether the unpredictability of the properties of a previously disclosed compound permit an otherwise obvious invention to be the subject of a second monopoly – Whether a second monopoly may be granted for a compound disclosed in the prior art on the basis that routine testing is required to enable the invention – Whether a selection patent should be exempt from the requirement that the claims be unambiguous.
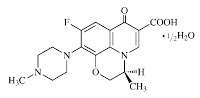
Daiichi Pharmaceutical Co., Ltd. (“Daiichi”) discovered ofloxacin, an antimicrobial drug used in the treatment of infections and obtained a patent for it in the early 1980s that expired in 2001. Ofloxacin is a racemic compound with a single chiral centre, a junction where there are two identical, three-dimensional molecules called enantiomers or optical isomers that are mirror images of each other. The right hand or dextro version is called (+) ofloxacin and the left or levo side is called (-) ofloxacin or “levofloxacin”. Further, the configuration of the enantiomers can be chemically described as being either “S” or “R”. After the discovery of ofloxacin, researchers at Daiichi experimented with techniques to isolate or resolve its enantiomers from the racemic compound. Their research indicated that the “S(-)” enantiomer had twice the antimicrobial activity, was less toxic, and was more soluble than the racemic compound. In 1986, Daiichi filed a patent application for levofloxacin which became Canadian Patent 1,304,080 (the “080” patent in 1992. This patent will expire in June 2009. Janssen-Ortho Inc. (“Janssen”), Daiichi’s Canadian licencee, markets and sells levofloxacin in Canada. In 2004, the Applicant, Novopharm Limited (“Novopharm”), obtained a notice of compliance from the Minister of Health, which allowed it to market its generic version of levofloxacin in Canada. In Daiichi’s prohibition proceedings launched under the Patented Medicines (Notice of Compliance) Regulations, Novopharm was successful in establishing that the 080 patent was void for obviousness and anticipation. When Novopharm began marketing its product, however, Janssen and Daiichi commenced infringement proceedings. In dispute was the validity of claim 4 of the 080 patent.
See also:
- Wipipedia: levofloxacin
- 2007.06.07 カナダ連邦控訴裁判所判決 Docket No. A-500-06
- カナダ連邦控訴裁判所判決時の第一三共プレスリリース: 2007.06.14「Canadian Levofloxacin Patent Upheld by Federal Appeal Court」
- 21世紀に伝えたい「新薬開発物語」日本製薬工業協会HPより


コメント