OSI pharmaceuticalsのpress release(SEC filing)によると、
EPO審判部は、OSIが保有する、ジペプチジルペプチダーゼIV(dipeptidyl peptidase IV(DP-IV、DPPIV又はDPP-4))に関する特許のひとつ(EP0896538: USE OF ACTIVITY INHIBITING DIPEPTIDYL EPTIDASE IV EFFECTORS FOR LOWERING THE BLOOD GLUCOSE LEVEL IN MAMMALS; ApplicantはOSIの子会社Prosidion)を無効と判断したとのこと。
本特許クレームは、糖尿病等を治療するためのDP-IV inhibitorの使用をカバーする機能クレームであり、2002年に大手製薬メーカー8社(Novartis, Bristol-Myers Squibb, Takeda, Pfizer, Boehringer Ingelheim, Glaxo, Novo Nordisk, Mitsubishi Tanabe)から異議を申立てられ、約6年間の歳月を費やして今回の審決に至った(Appeal No. T1151/04)。
参考:
- EP0896538 B1 Claims:
1. The use of activity lowering effectors of dipeptidyl peptidase IV (DP IV) or DP IV-like enzyme activity for the preperation of a medicament for the oral therapy of diseases which are based on glucose concentrations in the serum of mammals characteristic of hyperglycemia.
2. The use according to claim 1 for the preparation of a medicament for the prevention or alleviation of pathological abnormalities of the metabolism of mammals such as glucosuria, hyperlipidemia, metabolic acidosis and Diabetes mellitus.
3. The use according to claim 1 or 2, characterized in that inhibitors, pseudosubstrates, inhibitors of DP IV expression, binding proteins, or antibodies against said enzyme proteins or combinations of the designated effectors are used as activity lowering effectors of Dipeptidyl Peptidase IV (DP IV) or DP IV-like enzyme activity.
4. The use according to any one of the procedeing claims, characterized in that the effectors lower the activity of DP IV or DP IV-like enzyme activity with respect to GIP1-42 and/or GLP-17-36.
5. The use according to claim 4, characterized in that substrates are used as effectors.
6. The use according to any one of the preceding claims wherein a combination of effectors is used.
7. The use according to any one of the preceding claims, characterized in
that the effectors are alanyl pyrrolidide, isoleucyl thiazolidide, N-valyl-prolyl, O-benzoyl hydroxylamine.8. The use according to any one of the preceding claims, characterized in that the effectors are used in combination with at least one carrier substance such as glucose.
- OSI Pharmaceuticals press release: 2008.03.18 Pharmaceuticals Announces That the Prior Revocation of Its European DP-IV Patent Has Been Upheld
- Wikipedia: Dipeptidyl peptidase-4; Dipeptidyl peptidase-4 inhibitors

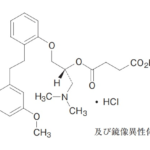
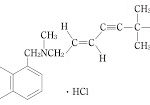
コメント
米国特許はどうするんでしょうね。
コメントありがとうございます。
ご指摘のとおり、米国でも下記のような機能クレームで特許(US 6,303,661 B1)となっています。ライセンスをあらかじめ得ておくか、それとも訴訟のリスクを背負うか、DPPIV inhibitorを開発する各企業の判断が問われますね。
US 6,303,661 B1
Claims:
1. A method for lowering elevated blood glucose levels in mammals resulting from food intake comprising administering at least one oral administration of a therapeutically effective amount of at least one inhibitor of Dipeptidyl Peptidase (DP IV) or of DP IV-like enzyme activity.
2. The method according to claim 1, wherein said at least one inhibitor is selected from the group consisting of alanyl pyrrolidine, isoleucyl thiazolidine, and N-valyl prolyl, O-benzoyl hydroxylamine.
3. The method according to claim 1, wherein said at least one inhibitor is administered in combination with at least one carrier substance.
4. The method according to claim 1, wherein said at least one inhibitor is administered in multiple administrations.
5. The method according to claim 1, wherein said amount is between 1.0 mg to 10.0 mg per kilogram of the inhibitor compound.
6. The method according to claim 1, wherein the mammals demonstrate clinically inappropriate basal and post-prandial hyperglycemia.
7. The method according to claim 1, wherein the administration is for the prevention or alleviation of pathological abnormalities of metabolism of mammals such as glucosuria, hyperlipidaemia, metabolic acidosis and Diabetes mellitus.
8. A method for lowering elevated blood glucose levels in mammals resulting from food intake comprising the administration of a therapeutically effective amount of an inhibitor of DP-IV enzyme activity comprising isoleucyl thiazolidine.
9. A method for lowering elevated blood glucose levels in mammals resulting from food intake comprising the oral administration of a therapeutically effective amount of an inhibitor of DP-IV enzyme activity selected from the group consisting of alanyl pyrrolidine and isoleucyl thiazolidine.