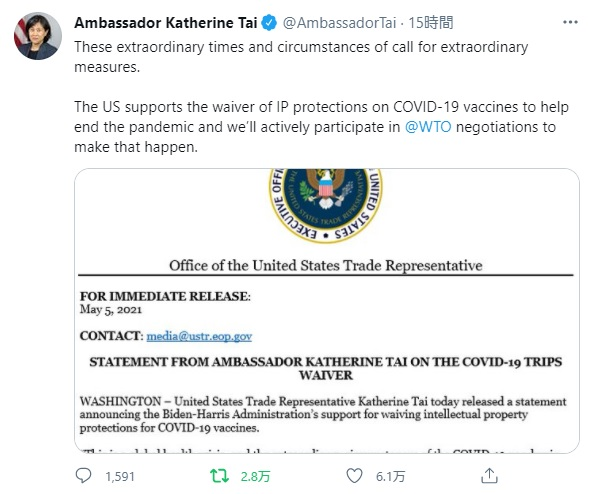
 2021年5月5日、米通商代表部(USTR)のタイ代表は、WTOでの議論(TRIPS協定において定められた知的財産保護に関する規程の適用除外/履行義務免除(waiver)を求める提案)を受けて、バイデン政権がCOVID-19ワクチンの知的財産権保護の放棄(前記waiver)を支持することを発表しました。その発表に対して各製薬業界団体は反対と失望の声明を出しています。
2021年5月5日、米通商代表部(USTR)のタイ代表は、WTOでの議論(TRIPS協定において定められた知的財産保護に関する規程の適用除外/履行義務免除(waiver)を求める提案)を受けて、バイデン政権がCOVID-19ワクチンの知的財産権保護の放棄(前記waiver)を支持することを発表しました。その発表に対して各製薬業界団体は反対と失望の声明を出しています。
バイデン政権の安全で効果的なワクチンをできるだけ早く多くの人々に届けるという目標には心から賛同しますが、知的財産権による保護を放棄することがパンデミックを終わらせるわけではありません。説得力を欠いた近視眼的な考えには到底賛同できません。
将来のパンデミックに対するイノベーションは誰が担うのでしょうか。
1.バイデン政権の声明
2021.05.05 The Office of the United States Trade Representative (USTR) press release: Statement from Ambassador Katherine Tai on the Covid-19 Trips Waiver
WASHINGTON – United States Trade Representative Katherine Tai today released a statement announcing the Biden-Harris Administration’s support for waiving intellectual property protections for COVID-19 vaccines.
“This is a global health crisis, and the extraordinary circumstances of the COVID-19 pandemic call for extraordinary measures. The Administration believes strongly in intellectual property protections, but in service of ending this pandemic, supports the waiver of those protections for COVID-19 vaccines. We will actively participate in text-based negotiations at the World Trade Organization (WTO) needed to make that happen. Those negotiations will take time given the consensus-based nature of the institution and the complexity of the issues involved.
“The Administration’s aim is to get as many safe and effective vaccines to as many people as fast as possible. As our vaccine supply for the American people is secured, the Administration will continue to ramp up its efforts – working with the private sector and all possible partners – to expand vaccine manufacturing and distribution. It will also work to increase the raw materials needed to produce those vaccines.”
Tweet from @AmbassadorTai:
These extraordinary times and circumstances of call for extraordinary measures.
The US supports the waiver of IP protections on COVID-19 vaccines to help end the pandemic and we’ll actively participate in @WTO negotiations to make that happen. pic.twitter.com/96ERlboZS8
— Ambassador Katherine Tai (@AmbassadorTai) May 5, 2021
2.米国研究製薬工業協会(PhRMA)の声明
2021.05.05 PhRMA press release: PhRMA Statement on WTO TRIPS Intellectual Property Waiver
WASHINGTON, D.C. (May 5, 2021) – Pharmaceutical Research and Manufacturers of America (PhRMA) president and CEO Stephen J. Ubl made the following statement after the United States Trade Representative expressed support for a proposal to waive patent protections for COVID-19 medicines:
“In the midst of a deadly pandemic, the Biden Administration has taken an unprecedented step that will undermine our global response to the pandemic and compromise safety. This decision will sow confusion between public and private partners, further weaken already strained supply chains and foster the proliferation of counterfeit vaccines.
“This change in longstanding American policy will not save lives. It also flies in the face of President Biden’s stated policy of building up American infrastructure and creating jobs by handing over American innovations to countries looking to undermine our leadership in biomedical discovery. This decision does nothing to address the real challenges to getting more shots in arms, including last-mile distribution and limited availability of raw materials. These are the real challenges we face that this empty promise ignores.
“In the past few days alone, we’ve seen more American vaccine exports, increased production targets from manufacturers, new commitments to COVAX and unprecedented aid for India during its devastating COVID-19 surge. Biopharmaceutical manufacturers are fully committed to providing global access to COVID-19 vaccines, and they are collaborating at a scale that was previously unimaginable, including more than 200 manufacturing and other partnerships to date. The biopharmaceutical industry shares the goal to get as many people vaccinated as quickly as possible, and we hope we can all re-focus on that shared objective.”
3.国際製薬団体連合会(IFPMA)の声明
2021.05.05 IFPMA press release: IFPMA Statement on WTO TRIPS Intellectual Property Waiver
Geneva, 5 May 2021 – The decision of the US administration to support a patent waiver for COVID-19 vaccines is disappointing. We are fully aligned with the goal to ensure COVID-19 vaccines are quickly and equitably shared around the world. But, as we have consistently stated, a waiver is the simple but the wrong answer to what is a complex problem. Waiving patents of COVID-19 vaccines will not increase production nor provide practical solutions needed to battle this global health crisis. On the contrary, it is likely to lead to disruption; while distracting from addressing the real challenges in scaling up production and distribution of COVID-19 vaccines globally: namely elimination of trade barriers, addressing bottlenecks in supply chains and scarcity of raw materials and ingredients in the supply chain, and a willingness by rich countries to start sharing doses with poor countries.
While the decision of the US administration does not address the real challenges in vaccinating the world, industry will not waver in its commitment to provide safe, effective and quality vaccines and therapeutics. We will continue to leave no stone unturned to further scale up manufacturing of COVID-19 vaccines, as no one is safe until everyone is safe. The international intellectual property system has given companies confidence to engage in more than 200 technology transfer agreements to expand delivery on COVID-19 vaccines based on unprecedented partnerships between vaccine industrialized and developing country vaccine manufacturers. The only way to ensure quick scaling up of and equitable vaccine access to all those in need remains pragmatic and constructive dialogue with the private sector.
4.欧州製薬団体連合会(EFPIA)の声明
2021.05.06 EFPIA press release: EFPIA statement on IP waiver for COVID-19 vaccines
Speaking about the US administration’s decision to support a proposal to waive patents on COVID-19 vaccines, EFPIA Director General Nathalie Moll said. “This short-sighted and ineffectual decision by the Biden administration puts the hard-won progress in fighting this terrible disease in jeopardy. While we wholeheartedly agree with the goal of protecting citizens around the world through vaccines, waiving patents will make winning the fight against the coronavirus even harder.”
She went on to say. “Increasing capacity to deliver doses to citizens around the world requires the skills and technical know-how of the vaccine developer to bring on-board partner manufacturing organizations. You simply cannot achieve this kind of capacity expansion by waiving patents and hoping that hitherto unknown factories around the world will turn their hand to the complex process of vaccine manufacture. A waiver risks diverting raw materials and supplies away from well established, effective supply chains to less efficient manufacturing sites where productivity and quality may be an issue. It opens the door to counterfeit vaccines entering the supply chain around the world. Capacity expansion is only achievable through voluntary, collaborative partnerships between the innovators behind each vaccine and expert manufacturing partners. All our focus should be on removing barriers to collaboration, ensuring the free flow of materials around the world and continuing the research effort.”
Since the very beginning of the crisis, Europe has been an engine room of COVID-19, research, development and innovation; from new diagnostics to mRNA technology used in the first approved vaccine. This research-based response, has given us the tools to fight the pandemic. A response built on an IP framework that incentivised innovators to explore these new technologies. Recognising the genesis of these vital tools, the European Parliament voted against the waiver on 29 April 2021 and the European Commission has remained consistent in their support for innovation as key to fighting the pandemic.
If approved by the WTO, the waiver would remove incentives for companies to continue research in to new variants, new diagnostics, treatments and vaccines to tackle the coronavirus. And at the same time, it would fail to increase the global capacity to manufacture COVID-19 vaccines. In addition, removing patents on COVID-19 vaccines would also negate any innovation-based response to future pandemics.
Nathalie Moll concluded by saying. “It is vital that Europe continues to champion medical innovation as the only permanent route out from under the shadow of the coronavirus, through its opposition to the waiver. As a global hub for vaccine manufacture, our focus should remain on creating the partnerships and investing in facilities to increase capacity to meet the needs to citizens across Europe around the world.”
5.バイオテクノロジーイノベーション協会(BIO)の声明
2021.05.05 BIO press release: Support of “TRIPS” Waiver Sets Dangerous Precedent
Dr. Michelle McMurry-Heath, president and CEO of the Biotechnology Innovation Organization (BIO), released the following statement in response to the White House’s support of waiving critical intellectual property rights for COVID-19 vaccines:
“We are extremely disappointed that the Administration has chosen to support waiving critical protections for American ingenuity and to delay the equitable delivery of needed COVID vaccines to people around the globe.
“Handing needy countries a recipe book without the ingredients, safeguards, and sizable workforce needed will not help people waiting for the vaccine. Handing them the blueprint to construct a kitchen that – in optimal conditions – can take a year to build will not help us stop the emergence of dangerous new COVID variants. The better alternative would have been to follow through on the President’s pledge just last week to make the United States the world’s “arsenal of vaccines”. This policy leads in the opposite direction.
“Today’s decision is especially disheartening after BIO put forward a list of policy solutions that would establish the COVID Global Strategy for Harnessing Access Reaching Everyone (SHARE) Program.
“The Global SHARE Program would ensure sufficient global supply of vaccines, ensure safe and expeditious global access to vaccines and therapeutics, and bolster ongoing efforts to strengthen and support healthcare systems in low-and middle-income countries in addressing COVID. It would accomplish these goals without compromising protections for intellectual property or further stretching limited global vaccine expertise to the breaking point.
“And all of this while we have yet to fulfill our existing commitment to the international COVAX vaccine donation program.
“BIO has warned on several occasions that the TRIPS waiver has the potential to drastically hinder existing efforts to scale up global manufacturing, disrupt efforts to equitably distribute the vaccines to every corner of the globe through COVAX, and further strain the global supply chain.
“Also of concern, this decision will disadvantage patients by undermining existing incentives to develop vaccines and therapeutics for future pandemics.
“As the U.S. participates in text-based negotiations moving forward with this ill-advised plan, BIO strongly encourages the Administration to:
- Prevent the expropriation of technology that has use beyond COVID vaccines which could be used to compete against American companies and workers in the future;
- Protect American companies from the coerced transfer of technology by foreign governments;
- Ensure that these actions do not impede global supply chains for existing facilities;
- Avoid any precedents that would work to undermine incentives to develop vaccines and treatments in future pandemics; and
- Ensure that the manufacturing of any vaccines is done in compliance with rigorous safety and manufacturing standards.
“The United States has unfortunately chosen to set a dangerous precedent with these actions. But how we negotiate with the WTO moving forward will be critical in mitigating this myopic decision and its effects on patients around the world.”


コメント
10月のWTOからの流れが記載されているとより良いのではないでしょうか。
【参考】WTOで南アフリカ及びインドから提起されたTRIPS協定における履行義務の放棄に関して・・・
過去記事: 「2020年、医薬系”特許的”な出来事を振り返る。」
1.COVID-19パンデミック危機に対するイノベーションのレジリエンス
https://www.tokkyoteki.com/2020/12/2020.html#toc1
2020.10.02 Council for Trade-Related Aspects of Intellectual Property Rights – IP/C/W/669: WAIVER FROM CERTAIN PROVISIONS OF THE TRIPS AGREEMENT FOR THE PREVENTION, CONTAINMENT AND TREATMENT OF COVID-19 – COMMUNICATION FROM INDIA AND SOUTH AFRICA
https://docs.wto.org/dol2fe/Pages/SS/directdoc.aspx?filename=q:/IP/C/W669.pdf&Open=True
ご指摘ありがとうございます。本文に以下の記載を追加等しました。
「WTOでの議論(TRIPS協定において定められた知的財産保護に関する規程の適用除外/履行義務免除(waiver)を求める提案)を受けて、」
2021.05.08 Albert Bourla Chairman and Chief Executive Officer, Pfizer
Today I Sent This Letter To Have a Candid Conversation With Our Colleagues About the Drivers of COVID-19 Access and Availability
https://www.linkedin.com/pulse/today-i-sent-letter-have-candid-conversation-our-drivers-bourla/
日本製薬工業協会(JPMA)の声明
2021.05.07 WTOにおける知的財産の放棄について
http://www.jpma.or.jp/event_media/release/news2021/210507.html
国際知的財産保護協会(AIPPI)の声明
2021.05.14 AIPPI Position Paper: TRIPS Agreement and the COVID-19 Waiver
https://aippi.org/aippi-position-paper-trips-agreement-and-the-covid-19-waiver/
2021.05.14 明治大学知的財産法政策研究所
高倉成男「知的財産と公衆衛生」(高倉成男・木下昌彦編『知的財産法制と憲法的価値』(有斐閣より2021年12月末刊行予定)に収録予定)の先行公開について
http://www.isc.meiji.ac.jp/~ip/IPandConst.html
高倉成男「知的財産と公衆衛生」(先行公開版)へのリンク (PDF)
http://www.isc.meiji.ac.jp/~ip/20210514takakura.pdf
・・・特許制度と公衆衛生のバランスを図るためには、強制実施権設定や特許保護義務免除でなく、公的資金による特許製品の買上げを提言されておられる。
少し時間が経ってしまいましたが、ドイツのマックス・プランク研究所の関係者有志が、声明を出しています。
https://www.ip.mpg.de/en/nc/research/research-news/covid-19-and-intellectual-property-10-arguments-against-a-waiver-of-ip-rights.html
ありがとうございます。リンク先が変更されたようなのでリンクを張り直しました↓
https://www.ip.mpg.de/en/research/research-news/covid-19-and-intellectual-property-10-arguments-against-a-waiver-of-intellectual-property-rights.html
西村もも子 知的財産権の一時放棄という決断と米国の国内政治
特許研究 PATENT STUDIES No.73 2022/3
https://www.inpit.go.jp/content/100874754.pdf
「先進国の知的財産制度自体が揺らぎを見せている今日,TRIPs 協定上の歪みを正すチャンスが,現在の新型コロナウィルスという異例の事態の下で到来したとみることができよう。」