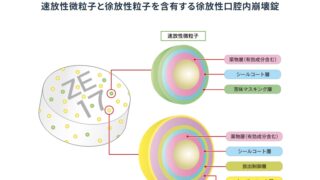
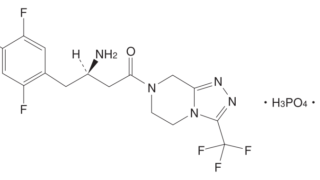
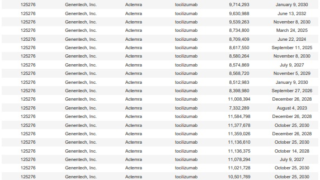
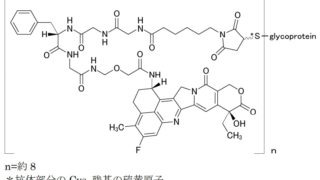
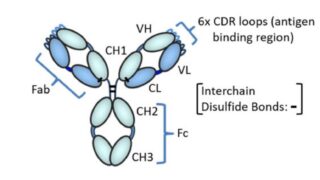
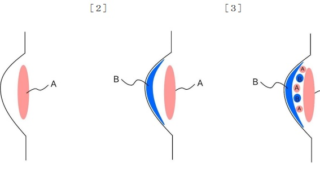
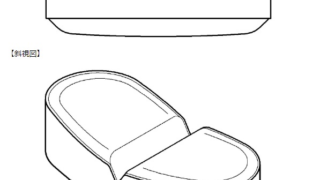
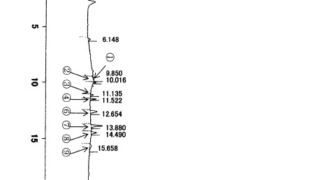
 *Case2024
*Case2024 2024.03.25 「X v. クリニジェン」 知財高裁令和5年(ネ)10103 ― 本件訴えは前件訴訟の蒸し返しであり信義則に反する不適法なものであるとして控訴を棄却した損害賠償請求事件 ―
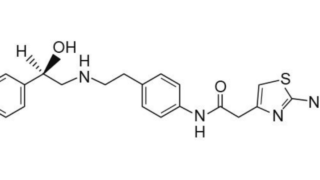
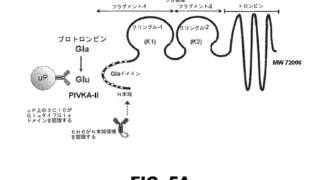
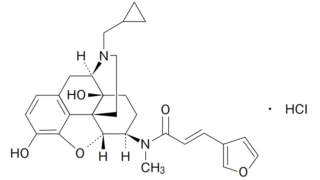
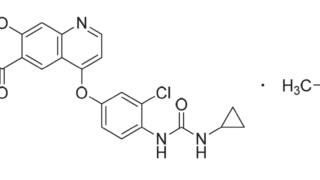
Summary クリニジェン株式会社が本件国内移行手続を執らなかった行為及び本件発明の権利化の機会を発明者である原告Xに与えなかった行為が譲渡契約上の債務不履行を構成すると主張して原告Xが同社に対し損害賠償の支払を求めた控訴審で、知財高裁は、本件訴えは前件訴訟(東京地裁令和3年(ワ)4655)の蒸し返しであって、訴訟法上の信義則に反する不適法なものであり、本件訴えを却下した原判決は相当であるとして...